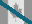Miglustat HCl
α-glucosidase inhibitor / Orally active ceramide-specific glycosyltransferase and α-glucosidase I and II inhibitor.1 Rescues trafficking-deficient F508del-CFTR in human airway epithelial cells via inhibition of ER α-glucosidases I and II.2 Pharmacological chaperone for glucocerebrosidase degradation.3 Clinically useful agent for Gaucher disease type 1.4 Stabilizes neurological disorders in Niemann-Pick disease type C.5
Biochemicals & reagents
210110-90-0
NB-DNJ
1) Platt et al. (1994), N-butyldeoxynojirimycin is a novel inhibitor if glycolipid biosynthesis; J. Biol. Chem., 269 8362 2) Noel et al. (2008), Parallel improvement of sodium and chloride transport defects by miglustat (n-butyldeoxynojyrimicin) in cystic fibrosis; J. Pharmacol. Exp. Ther., 325 1016 3) Abian et al. (2011), Therapeutic strategies for Gaucher disease: miglustat (NB-DNJ) as a pharmacological chaperone for glucocerebrosidase and the different thermostability of velaglucerase alfa and imiglucerase; Mol. Pharm., 8 2390 4) Serratrice et al. (2015), Switching from imiglucerase to miglustat for the treatment of French patients with Gaucher disease type 1: a case series; J. Med. Case Rep., 9 146 5) Karimzadeh (2013), Effects of miglustat on stabilization of neurological disorder in niemann-pick disease type C: Iranian pediatric case series; J. Child Neurol., 28 1599
-20°C
TARGET: Glycosylation; Chaperone -- PATHWAY: Carbohydrate metabolism; Sphingolipid metabolism -- RESEARCH AREA: Neuroscience