Background
Bruton’s agammaglobulinemia Tyrosine Kinase (BTK) is an important regulator of B-cell development and signaling (1), and as such, has been investigated as a target of inhibition for the treatment of B-cell malignancies, autoimmune disorders, and inflammation (2). The activity of BTK is regulated by a variety of mechanisms, including membrane translocation and phosphorylation. Key in the activation process is phosphorylation of two tyrosine residues, Y551 and Y223 (1). It is generally believed that Y551 of BTK is phosphorylated by a Src family kinase, likely Lyn, which subsequently leads to autophosphorylation of the Y223 site (3, 1). However, BTK has also been shown to autophosphorylate at the Y551 site, at least in vitro (4).
BTK [C481S] is a variation of wild type BTK where the cysteine at amino acid 481 has been mutated to serine. The clinical compound PCI-32765 (ibrutinib) irreversibly binds BTK by reacting with the cysteine at position 481 (5). As such, ibrutinib is predicted to exhibit reduced binding and potency toward BTK [C481S] compared to the wild type protein. Consistent with this prediction, the BTK [C481S] mutant has recently been reported in ibrutinib-resistant patients (6). Wild type BTK is also sensitive to dasatinib which has a different MOA from ibrutinib. Two gatekeeper BTK mutants, BTK [T474A] and BTK [T474M], are resistant to dasatinib but sensitive to ibrutinib to different degrees.
ClariCELL™ assays were used to quantify autophosphorylation of human full-length wild type BTK, BTK [C481S], BTK [T474A] and BTK [T474M], and the cellular potencies of ibrutinib and dasatinib on modulation of autophosphorylation events.
Results
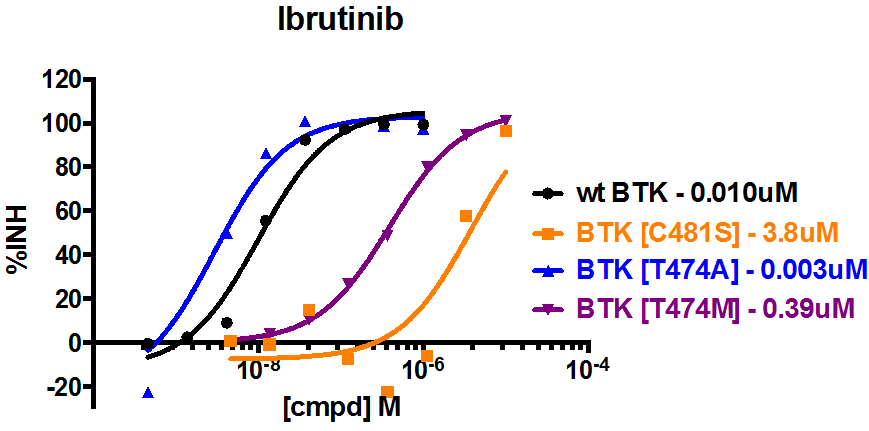
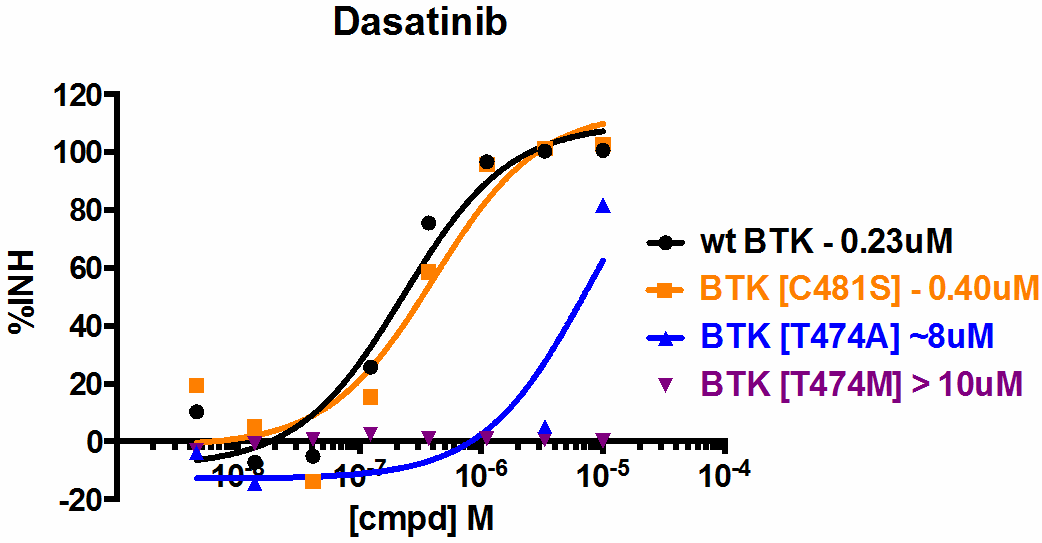
As expected, the BTK [C481S] mutant is resistant to ibrutinib while the wild type BTK and gatekeeper mutants show sensitivity. Interestingly, the BTK [T474A] is as sensitive to ibrutinib as wild type BTK, but the BTK [T474M] mutant is moderately (10-20 fold) less sensitive to ibrutinib. On the other hand, in the presence of dasatinib, both BTK and the BTK [C481S] mutant exhibit sensitivity while the BTK gatekeeper mutants are resistant. Together, results from these assays highlight important mechanism of action and binding differences between the tested compounds.
References
- Mohamed, AJ et al., Bruton’s Tyrosine Kinase (Btk): function, regulation, and transformation with special emphasis on the PH domain, (2009) Immunol. Rev., 228:58-73.
- Vargas, L et al., Inhibitors of BTK and ITK: State of the new drugs for cancer, autoimmunity and inflammatory diseases, (2013) Scand. J. Immunol., 78(2):130-9.
- Rawlings, DJ et al., Activation of BTK by a phosphorylation mechanism initiated by SRC family kinases, (1996) Science 271:822–825.
- Dinh, M et al., Activation mechanism and steady state kinetics of Bruton’s Tyrosine Kinase, (2007) J. Biol. Chem. 282:8768-8776.
- Pan, Z et al., Discovery of selective irreversible inhibitors for Bruton’s Tyrosine Kinase, (2007) ChemMedChem 2:58-61.
- Woyach, JA et al., Resistance mechanisms for the Bruton's Tyrosine Kinase inhibitor ibrutinib, (2014) N Engl J Med., 370(24):2286-94.
